NATS 101 - Intro Global Change
- Spring 2006
Group Activity 1 -
Element Summaries
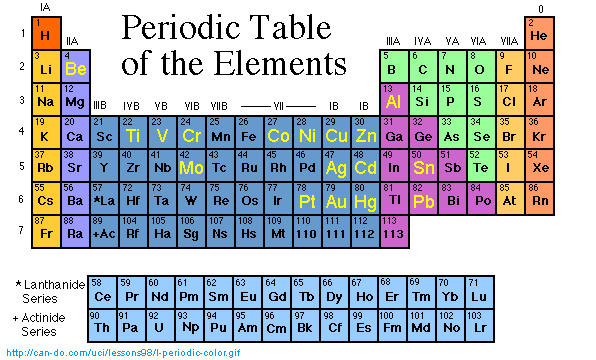 *Click* on a highlighted element symbol to
jump to the summary, or scroll in order of the class groups.
Course home page
*Click* on a highlighted element symbol to
jump to the summary, or scroll in order of the class groups.
Course home page
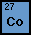 |
Cobalt - Group 1 |
|
|
Cobalt, an element found on the periodic table of element
is most commonly produced as a by-product of other elements such
as nickel, copper and iron. The atomic number of Cobalt is 27
and the atomic weight is 58.933200. The most abundant isotope
is 59. The density of Cobalt is 8.9 g/cm3. It is metallic gray
in color and can be found in many forms including foil, pieces,
powder, rod, and wire.
Each year approximately 35,000 tons of Cobalt is mined each
year. There are no Cobalt mines in the United States, although
the US is one of the largest consumers of the element. The United
States produces only 11% of the amount of Cobalt that the country
uses; it comes from areas of Colorado and the South West. Researchers
also believe that Cobalt can be found at the bottom of the Pacific
Ocean, near the Hawaiian Islands. The countries that produce
large amounts of Cobalt include Australia, Canada, Zambia, Russia,
Cuba, and Morocco. The countries that produce Cobalt are mining
intensive and tend to be dry and rocky. Cobalt can be found in
open pit mines, but not as free metal. Over the years the cost
of Cobalt has varied from under $3.00 all the way up to about
$50.00. Right now the average cost of Cobalt is $21.00 for 100
grams.
Cobalt can be found in batteries, vitamin B-12, it is in drying
agents of paint, nuclear weapons and gas turbine engines. Cobalt
is also used to fight cancer through radiotherapy. When humans
or the environment are exposed to too high levels of Cobalt there
can be potential risks including but not limited to cancer, cardiovascular
disease, lung and breathing problems, vision problems, along
with thyroid damage. |
Back to
top
 |
Nickel - Group 2 |
|
|
The element nickel with the symbol of Ni has an atomic
number of 28. Nickel is a mixture of ferrous and nonferrous metal
properties and is a transition element. The most common isotope
has 58 neutrons. Nickel is mined through strip mining with cause's
erosion of top soil, in turn degrading the ecosystem. Nickel
can be very toxic if inhaled in dust form, and skin contact can
result in rash and repeated contact results in an increase chance
of lung and skin cancer.
65% of all the nickel mined is used to make stainless steel.
Other products with nickel include rechargeable batteries, U.S.
and Canadian coins, kitchen ware, and wires. Canada produces
about 30% of the worlds of nickel, and Russia contains about
40% of the worlds known supply. 100 million tons of nickel is
produced each year. The production of nickel is on an upward
trend; there has been a 30% increase in nickel production between
the years of 1993-1998. There has been a 22% of nickel production
in Japan for its increasing demand to make products such as robotics,
smart wires, and high communication devices.
Nickel is a white-silver shiny metal. It is hard and ductile
and belongs to an iron group. Often is accompanied by cobalt
since both are found in meteoric iron. Mostly its value comes
from the alloys it forms. Nickel is also magnetic. Its atomic
mass is 58.6934 grams per mole, and its melting point in 1455
degrees Celsius. Most of the Earth's nickel concentration is
found in the Earths core, based on geophysical evidence. |
Back to
top
 |
Beryllium
- Group 3 |
|
|
?Got Beryllium?
In 1828 more then a century ago the metal beryllium was isolated
by Friedrich Wölhler and A. Bussy. Both chemists discovered
the alkaline earth metal independently through the action of
potassium on beryllium chloride. Today Beryllium is found in
over 30 mineral species but the two most common are beryl and
bertrandite. Beryl and Bertrandite are the most important commercial
sources of the element. The metal is prepared by reducing beryllium
fluoride with magnesium metal. However beryllium metal did not
become readily available to the industry until the late fifties.
The estimated crustal abundance of beryllium is 2.8 milligrams
per kilogram and the estimated oceanic abundance is 5.6 x 10?6
milligrams per liter. The over all abundance of beryllium is
relatively low and due to a complex extraction process it is
not produced in laborites. As a result beryllium is produced
commercially and can be purchased in various amounts.
Beryllium can be purchased in an assortment of different forms.
Beryl is colorless in pure form but when it is mixed with other
minerals will demonstrate a splendid color variety. In fact aquamarine
and emerald are precious forms of beryl and are readily useful
in the commercial market. Beryllium has many desirable properties.
It is one of the lightest of all metals and has one the highest
melting points of the light metals. At ordinary temperatures
beryllium resists oxidation and such properties make it very
useful in the industrial world. The ability to resist large amounts
of heat makes it useful for the production of spacecrafts, missiles,
aircrafts, non-sparking tools, and communication satellites.
It has also been known for being more elastic then steel and
can also be used in X-ray tube windows and watch springs. Beryllium
can be used for many things but contains many toxins and should
be handled with great care.
Beryllium and many of its compounds are poisonous and should
not be tasted or ingested. Individuals that exposed to these
toxins often develop lung and skin disease. Exposure usually
occurs in the mining, extraction, and in processing of the metal.
According to the U.S. Department of Labor Occupational Safety
and Health Administration, ?controlling the exposure to beryllium
can be done through engineering controls, administrative actions,
and personal protect equipment? (OSHA). Harmful effects of beryllium
occur depending on how much you are exposed to and for how long.
The average Beryllium metal price has steadily increased since
the later seventies and is relatively the same price as it was
in the late fifties. As telecommunications companies grow and
a need for industrial materials for spacecrafts increase so will
the production of beryllium. The trend will match the socio-economic
situations and may change rapidly in the future depending on
many political factors. As always resources that are harmful
to humans and the environment should be lessened and hopefully
eliminated in the future.
Work Cited
"Beryllium: Safety and Health Topics." Occupational
Safety and Health Administration. U.S. Department of Labor. Jan
2006 <http://www.osha.gov/SLTC/beryllium/>. |
Back to
top
 |
Gold - Group
4 |
|
|
Gold, whose periodic symbol is Au, has an atomic number
of 79; although its most abundant isotope has 197 neutrons. The
abundance of gold in the earth?s crust is .005 parts per million.
This element is not frequently in chemical reactions. Gold has
7 oxidation states, Au ^(-I), Au^(0), Au^(I), Au^(II), Au^(III),
Au^(V), and Au^(VII).
Gold occurs in veins and alluvial plains of the planet, specifically
in back arc basins and magmatic arcs. In granite, gold has an
average concentration of .00000002 percent. With gold mining
this mineral is recovered from its ores by smelting and amalgamation,
among other processes. South Africa, the United States, and Australia
are the leading gold mining countries (in that order). In the
United States, gold mines are most abundant in South Dakota and
Nevada. In addition to the valuable metal, gold mining produces
an array of environmental problems. For example, the Zortman-Landusky
gold mine in Montana struggles with cyanide spills, causing contamination
of surface and groundwater. Mercury is also often found when
mining gold, which poses threats to the surrounding lands and
people.
Historically, gold has played a large role in many nations? economies.
Today however, its role is diminished and thought to be largely
psychological. People value gold because it has traditionally
been associated with wealth. The currency of the United States
is no longer backed by gold, but by the faith and credit of the
country. On the market in 2000, gold sold for $279.77 per ounce.
Presently gold has a two tiered pricing system, which resulted
from the 1980 Gold Crisis, at which point prices significantly
rose. |
Back to
top
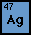 |
Silver -
Group 5 |
|
|
The element silver is called Ag on the periodic table
with an atomic number of 47. It's most abundant isotope is Ag
107 with an electron structure of 2, 8, 18, 18, 1. Silver forms
chemical bonds like hydrides, oxides, and chlorides with oxidation
states ranging from zero to three. Zero being the minimum oxidation
state and three being the maximum with a common oxidation state
of one. The abundance of the element in the earth's crust is
small compared to other elements and none in the mantle. Some
of the primary minerals that are contained in ore's with silver
are sulfur, arsenic, antimony, and chlorine. Many of these ores
are found in geological settings with climates that are warm
somewhat mountainous terrain as well as places that don't tend
to reach very cold temperatures. Somewhere between 900 million
ounces of silver is produced each year with Mexico being the
biggest producer with countries such as Peru, Australia and China
following after. The geology is linked to these countries because
they are all somewhat warm mountainous climates.
Silver is mined by open pit and underground methods as well,
however mainly open pit. Once mined the silver is crushed and
ground to make the element more refined. There are no current
silver mines in the us, however back in the 1800's silver was
mined in states such as Colorado, California, and Nevada. There
have been no really significant shifts in the trend of silver
production. Because it was a popular metal back hundreds and
hundreds of years ago silver has been on more of a downward trend
concerning its value and demand in the economy. Recently silver
prices have been on a slow upward trend and prices have ranged
from four dollars to as much as almost seven dollars from 2000
to today. If the United States was to control this production
it would really hurt the world economy as a whole. Since the
US doesn't have any mines that are still open to this day they
would need to have much of the silver shipped to them and then
transported and traded with other countries. This would cause
and uprising in silver prices and probably a pretty big crises.
Silver itself has no extremely serious affects. Yet, silver
ore experienced in large amounts can be carcinogenic or cancer
causing, however, not all forms of silver ore are this way. One
might see that silver is used in many every day objects such
as jewelry, silverware, photography, and industrial applications
such as cars and the things in them. Silver tends to be a one
of a kind sort of metal, and would be hard to be replaced. Many
of the things silver is used for is this way because silver is
a very tough and hard to break metal. Things such as aluminum
or tin would never be able to withstand some of the pressures
and hard tasks that a thing such as silver withstands. Not to
mention that they wouldn't be as pretty either. |
Back to
top
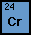 |
Chromium
- Group 6 |
|
|
Chromium is number 24 on our Periodic Table of Elements.
It can be found in the group 6 transition metals. This metal
was discovered by Louis Vauquelin of France in 1797. Vauquelin
discovered that he could isolate metallic chromium by heating
the oxide of CrO3 in a charcoal oven. Chromium is a steel-gray,
lustrous, hard metal that takes a high polish. Chromium also
melts (but with difficulty), and tarnishes.
Chromium is unstable in oxygen, but is very commonly used
in oxidation states. The most common oxidation states of chromium
are +2, +3, and +6. Chromium compounds of oxidation state 6 are
powerful oxidants. Chromium is used to make stainless steel,
chromium is also used to make heat-resisting steel and have strategic
military applications. It also gives rubies and emeralds their
color.
Geologists estimate that there is about 11 billion tons of
chromium ore in the world that could be mined. However, chromium
does not occur free in nature. The only ore of chromium is the
mineral chromite. United States chromium consumption is equivalent
to about 14% of all the chromite ore mined each year. 80% of
the worlds production of the metal comes from India, Kazakhstan,
Turkey, and southern Africa. Chromium is a very important metal
and is used daily in our society. |
Back to
top
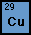 |
Copper -
Group 7 |
|
|
An Introduction to Copper
Copper is an element that surrounds us in our daily lives,
from the "copper" penny, which is no longer made purely
of copper, to countless household appliances and electronics,
to the Statue of Liberty. Copper is abbreviated Cu on the periodic
table, and its atomic number is 29. There are two stable isotopes
of copper, Cu-63 with 34 neutrons and Cu-65 with 36 neutrons.
The electron configuration for copper is 1s2 2s2p6 3s2p6d10 4s1.
As a metal, copper loses outer electrons comparatively easily
in chemical bonding, but does not gain them freely. Copper oxidizes
easily, forming bluish-green salts, and has three oxidation states:
the less stable Cu+1, the more stable Cu+2, and Cu+3. Throughout
the world, copper is the twenty-sixth most common element, making
up approximately .7 x 10-5% of the earth's crust and found in
a variety of minerals. There are two main types of copper ores,
sulfide ores, such as covellite, chalcocile, and enargite, and
oxide ores, such as tenorite, malachite, azurite, cuprite, chrysocolla,
and brochanite. 90% of the world's economically workable copper
ores are sulfide ores, many of which have a volcanic origin.
Copper has been manipulated for human use as far back as 8700
B.C. Historically, major producers have been the Roman Empire,
China in the Middle Ages, and Sweden in the more recent past.
Today the main producers are Chile, the United States-and, within
the United States, southwestern states like Arizona and Utah-and
Indonesia. Copper can be mined in a variety of ways, the most
common of which is open-pit mining. To be economically viable
to mine, a copper ore should have a concentration of 0.4 to 1.0%.
However, due to the decreased availability of high concentrations
of this important metal, new technologies are finding ways to
mine lower and lower concentrations all the time. World demand
for copper has been steadily increasing, a reflection of its
great usefulness in electronics, plumbing, roofing, and a variety
of other fields. World production of copper has been on the decline,
as its concentration dwindles.
There are some problems associated with copper. As with all
mining, copper mining can be devastating to the environment.
Open pit mining changes the character of land, displacing regional
plant and animal species. Insufficient pollution control can
also have a negative impact on local water. Some negative health
issues have been tied to copper as well. Although toxic levels
of copper rarely exist, and copper in appropriate doses is vital
to the human diet, extreme exposure to copper in the air can
produce what is known as "metal fever." This flu-like
malady lasts approximately two days and involves headaches, nausea,
dizziness, etc. Intentionally high uptakes of copper can lead
to liver or kidney damage, or possibly even death. These cases
are unusual, however. For thousands of years, copper has surrounded
us as an element highly useful in countless aspects of human
life. |
Back to
top
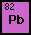 |
Lead - Group
8 |
|
|
Lead is Awesome
There is much to be known about lead. For instance, lead has
the symbol Pb on the periodic table because the Latin word for
it is plumbum. Its atomic number is 82 and in its most abundant
isotope there are 125 neutrons. This makes the mass number of
this isotope 207. Lead’s electron structure goes from the
1s orbital all the way to 6p2 (there is no easy way to type this
that I know of). The last thing you need to know about the chemical
properties of lead is that it has oxidation states of 2 and 4.
32,800,000 tons of lead is produced worldwide each year. The
primary producers of lead, in increasing order, are: Mexico,
Peru, the United States, Australia and China at number one. Lead
tends to be found in places with rocky terrain and near elements
such as zinc, silver and copper. Lead is mined in subsurface
mines and Missouri is responsible for 90% of the lead mined in
the United States. The actual composition of what is taken out
of the mines is PbS. The sulfate is "roasted" and sulfur
is released, leaving only the lead. There have been many shifts
in the production of lead throughout its history. The two biggest
shifts occurred when mass transportation popularized and when
the Environmental Protection Agency (EPA) discovered the harmful
affects of lead in the environment. Both world production and
U. S. production of lead has decreased 1% over the past year.
Lead is used in many products all around the world. It is
used in batteries and power sources, some paints, glazes and
radiation shields, just to name a few. Unfortunately, lead also
creates many environmental problems. It causes pollution in water
and the air, and in turn, kills wildlife. Lead can also poison
food. It also causes health problems when it is in high concentrations.
Lead can cause blood pressure to raise, decline fertility rates,
cause miscarriages and many other serious side affects. Lead
seems to be an element that people all around the world rely
on. As long as the use and production of lead is controlled,
it is a fantastic resource to have. |
Back to
top
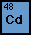 |
Cadmium -
Group 9 |
|
|
The atomic number of Cadmium is 48. The most abundant isotope
has 64 neutrons. The electron structure is 2.8.18.18.2. The most
common oxidation state is +2. An important chemical bond that
Cadmium creates is the compound of Cadmium Oxide CdO. Most of
the Cadmium is found in the crust of the earth and the upper
part of the mantel. Since the crust is not that thick it is easy
to mine for Cadmium. There must be at least .3% of cadmium within
the ore of sphalerite (ZnS). The ore contains Zinc and Sulfur.
Zinc is silver in color and very brittle while sulfur is yellow
in color but also brittle and hard. Cadmium is mined usually
underground where it is found in pits. However due to plate tectonics,
zinc ores can be found in volcanic settings near fault lines.
Approximately 20,000 tons of cadmium is produced each year worldwide.
The main countries of production are Japan, Canada, Mexico, China,
Korea, and the United States. The countries in Asia have an advantage
due to geology because they have more fault lines where the ores
can show up due to eruptions. To mine cadmium, one has to mine
zinc and process the zinc to obtain the cadmium. To mine zinc,
allows the ore to be mined steadily from bottom to top and backfilled
with waste rock. Once the zinc is minded, the cadmium can be
uncovered and used for production. Most of the production of
cadmium in the United States is in Tennessee. The amount of production
has declined because of the health risks involved with the exposure
to cadmium. The United States government had set up laws and
regulations to limit the production. However, with the recent
idea of recycling the cadmium batteries, it is safer and more
efficient. The price of cadmium is 32 cents per kg. If the United
States controlled all the production of cadmium we would be on
top of the economic market. Since Cadmium is used to make Ni-Cd
batteries it would put us at and advantage. These batteries are
found in all major electronics and appliances that are safe to
use and are sold at for a cheap price.
Environmental problems that would arise would be the contamination
of cadmium in the air. However, the effects of the cadmium in
the air is not dangerous and has little presents, the problems
in mining would not be an issue. Cadmium is used primarily in
Ni-Cd batteries. In addition to batteries, cadmium is found in
coating and red or yellow pigments for plastics and ceramics.
One would find this element in their house because it is found
in consumer products such as cell phones, power tools, portable
computers, household applications, and toys. The cadmium used
in these batteries is very low cost and work well with appliances.
Health problems that arise in cadmium are mainly by the digestion
of cadmium in food products. Cadmium does not have much presents
in the air one breathes, however, cigarettes contain cadmium
which increases the amount in one?s body. The health effects
of digesting cadmium are seen in the kidney, bones, and lungs.
This can result in kidney and lung failure later in life. However,
cadmium is becoming a safer element each year. Cadmium in the
Ni-Cd batteries are being recycled to cut down the use of mining
for more and to cut the costs around the world there are these
recycling centers to help reuse and become more efficient with
Cadmium. Since Cadmium is becoming safer to use and it is cheaper
to produce this element is the most suited to do the job. |
Back to
top
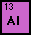 |
Aluminum
- Group 10 |
|
|
Aluminum is the 3rd most abundant element in the earth?s
crust. Aluminum is in 7.3% of the earth?s mass and about half
its volume. Aluminum does not exist on its own, but only in consistent
conditions with other minerals. There is some aluminum in the
lower mantle but aluminum bearing minerals are one of the most
plentiful in the upper mantle. The most common ore of aluminum
is bauxite. Bauxite is a reddish hard, clay substance. Aluminum
is created mostly in a tropical or subtropical climate and so
most bauxite deposits are found in these environments. There
are large blanket deposits of Aluminum in Western Africa, Australia,
South America, and India. 80% of bauxite comes from these as
they are the easiest to mine because they are typically just
beneath the topsoil. Bauxite, however, can be buried up to 70
meters underground. Pocket and interlayed deposits are found
elsewhere. In the U.S., bauxite can be found in Arkansas in detrital
deposits. Underground deposits are found beneath layers of carbonic
rock and require dewatering shafts to be drilled before mining.
Unlike base metals, bauxite processing is fairly simple. It is
simply washed, ground, and dissolved to allow aluminum to sink
to the bottom and be removed. Aluminum, also, is found in other
silicates and hydroxides.
The environmental effects of aluminum are interestingly complex.
Increasing usage of aluminum has the potential to reduce greenhouse
gas emissions but the production of aluminum emits greenhouse
gas and has high energy consumption. Recycled aluminum, however,
is more beneficial as it can save up to 95% of those greenhouse
gas emissions from primary production. Demand for aluminum has
been steadily increasing, for example, the automotive industry?s
demand for primary aluminum has doubled in the past 8 years.
However, the recycling of aluminum is increasing heavily as well.
Aluminum production began about 146 years ago, but its production
has far surpassed that of all other non ferrous metals. As the
benefits of aluminum have become more apparent, its production
has increased. For example from 1946 to 1999, aluminum production
increased from 681,000 tons to 24 million tons. The majority
of aluminum is currently produced using hydropower and is expected
to be produced this way in the future. This energy source has
some negative environmental effects but the industry is working
to reduce these and other effects including emissions and land
damage from the extraction of bauxite.
Aluminum is a strong but light metal and, therefore, has many
uses. The automotive industry is using aluminum extensively due
to its strength, kinetic energy absorption, and anti-rust quality.
Most freight trains are made of this element and high speed passenger
trains are beginning to use aluminum as well. Many aluminum products
are used in the construction of homes because of its anti-corrosion
quality which makes it almost maintenance free. Earthquake zones
use aluminum in construction for its strength and lightness.
Aluminum is, also, used extensively in cookware and packaging.
Aluminum creates a barrier against UV light, odors, and bacteria
when used in packaging. It, also, transmits conducted heat but
reflects radiant heat which has caused its extensive use in baking
and home insulation. |
Back to
top
 |
Platinum
- Group 11 |
|
|
Platinum is number 78 in the periodic table of elements
and has the symbol of Pt. The number of neutrons in the most
common and stable isotope is 117 with an electron structure of
1s2 2s2p6 3s2p6d10 4s2p6d10f14 5s2p6d9 6s1. It is fairly resistant
to chemical attack, has excellent high-temperature characteristics,
and stable electrical properties. Common oxidation states of
platinum include +2, +3, and +4. Normal levels found in the earth?s
crust are on the order of 5 µg/kg. Platinum is an extremely
valuable mineral and is usually considered to be about twice
the price of gold. As of January 27, 2006 the price of platinum
was $1055 per ounce (with 1 ounce equal to 0.0283495231 kilograms).
Platinum is a heavy, malleable, ductile, precious grey-white
transition metal and is resistant to corrosion and occurs in
some nickel and copper ores.
In 2005 around 6.59 million ounces were mined with 78% of
the total platinum being found in South Africa. North America
accounts for just 5% of the platinum mined. Open-pit mines and
a few underground mines are located in southern Montana, Alaska,
and Ontario, Canada. Platinum occurs in alluvial placer deposits
(where soil land is deposited by a river or other running water
and this leads to an open-pit style of mining where the minerals
are extracted from the surface of the earth without tunneling)
and is produced commercially as a by-product of nickel ore. The
large quantities of nickel ore processed make up for the small
platinum content which is only 0.5 parts per million in the ore.
The amount of platinum in the earth?s crust is unknown. It is
the difficult to mine platinum because of its low occurrence.
It is estimated that in order to produce a single ounce of platinum
a volume of 7 to 12 tons of ore must be processed. The environmental
risks of mining platinum are similar to mining gold. Since platinum
is mined in alluvial placer deposits, digging is minimized. However,
the results of processing large amounts of ore can lead to over-mining
certain areas. The element of platinum is toxic to some species
and platinum salts are known allergens at concentrations found
in occupational settings.
Platinum remained a scarce element until 1820 when deposits
were discovered by chance in the Ural Mountains of Russia. This
became the principal source of platinum for the next 100 years.
In 1925 two deposits with each around 100 kilometers in length
were discovered in South Africa and are now known as the Bushveld
Igneous Complex. Its mines today provide over three quarters
of the world?s platinum output. South Africa, in 2000, acquired
47% of its total value of exports through minerals, including
a large amount of platinum. If South Africa, or any other country
for that matter, could control the production of platinum it
would boost its economy dramatically and lower the other platinum-producing
country?s economies by eliminating platinum as a source of revenue.
The high demand for platinum is steadily increasing because of
the small amount of the supply and because of the numerous uses.
One of the principal uses of platinum is in the petroleum industry,
where platinum catalysts were introduced to increase the octane
rating of gasoline and to manufacture important primary feedstock
for the plastics industry. Platinum is also in high demand for
jewelry. It is also used in catalytic converters which convert
the noxious gases in vehicle exhausts into harmless substances.
Platinum is also capable of intercalating into DNA and is a chemotherapeutic
agent. Finally, platinum is used in platinum resistance thermometers
and in electrodes for use in electrolysis. If platinum poses
environmental problems similar metals such as gold could serve
in the place of platinum in some instances because of their similar
properties. |
Back to
top
 |
Molybdenum
- Group 12 |
|
|
Molybdenum, number forty-two on the atomic table, is a gray
metal that is used for foil, sheeting, wire, mesh, aircrafts,
oil pipelines, missiles, and in steel. Because of its strength,
molybdenum was used greatly during World War II. It has fifty-eight
neutrons in its largest isotope and fifty-six in its most abundant
isotope. Molybdenum is known for forming covalent bonds and its
oxidation states are 2,3,4,5, and 6.
Its abundance is .2 log in the Earth?s crust and is found
in wulfenite, powellite, and most commonly, molybdenite. However,
molybdenum is mined directly and as a byproduct of copper mines.
Most of these mines are in the United States in Montana, Idaho,
Colorado, New Mexico, Utah, and Arizona with the Phelps Dodge
Corporation as the largest provider. The other largest mining
country is Chile producing over 163,000,000 metric tons per year
in combination with the United States. The areas of mining in
both countries are open mountainous regions and the mines are
usually open cast pits or underground block caves. The price
has increased from $2 per pound in the year 2000 to roughly $40
per pound in 2005, thus showing an upward trend.
Although molybdenum has a very low toxicity, some cases have
been reported when large quantities have been ingested and no
cases of exposure harm have been reported. However, animals that
ingest molybdenum upset the balance of copper in their livers
are can suffer from emaciation, diarrhea, convulsions, blindness,
osteoporosis, and heart failure, but symptoms vary based on the
animal. There are substitutions for molybdenum, should its effects
upset the environment. These include tungsten, vanadium, chromium,
and boron. |
Back to
top
 |
Tin - Group
13 |
|
|
|
Back to
top
 |
Zinc - Group
14 |
|
|
The Wonderful World of Zinc
The symbol for Zinc is Zn, and its atomic number is 30 with
a weight of 65.409 (4) g. Zinc is the 23rd most abundant element
in the earth?s crust and comprises an estimated 0.004% of the
Earth?s crust. The abundance of zinc in the Universe is 300 ppb
by weight, crustal rocks 79000 ppb and human 33000 ppb. These
weights are given in (parts per billion). Sphalerite ZnS, zinc
sulfide, is and has been the main ore mineral in the world. In
the United States about two-thirds of zinc is produced from ores
(primary zinc) and the rest from scrap and residues (secondary
zinc). Zinc?s standard state is solid at 298K and is bluish pale
gray in colour. Zinc is available in many forms including dust,
foil, granules, powder, pieces, small activated powder,shot and
a mossy form. Most zinc production is based on sulfide ores.
These are made fired in industrial plants to form zinc oxide,
ZnO. This may be reduced with carbon to form metal. Pure zinc
may be formed from crude zinc by zone refining. Zinc has a self-healing
mechanism in it. The zinc coating gives way slowly by galvanic
action to protect the base steel. The concentration of zinc in
average granite is 0.005% and collected zinc concentration is
usually done at the mine site, prior to reaching the zinc processing
plant. The concentration includes crushing and flotation techniques.
At the plant, the zinc is reduced using distillation or electrowinning
process.
Ore mineral deposits have been associated with plate tectonics.
Deposits of iron,copper and zinc sulfides are forming within
rift valleys along the divergent oceanic plate boundaries. Zinc
bodies are proposed to be associated with convergent boundaries,
where they may be reformed then concentrated in the overlying
continental plate as the oceanic plate is reduced. Huge sulfide
deposits may be associated with island volcanism, and faulting
has been recognized as providing a charge for the accumulation
of metal-rich fluids. Ores containing zinc are geologically widespread
and many ore bodies are still waiting development. The major
zinc ore deposits are volcanic hosted sulfides, sediment hosted
sulfides, Mississippi Valley Type, (MVT) carbonate-hosted deposits,
intrusion related zinc ore deposits and "Broken Hill type,"
ore deposits. Zinc ores are being mined in more than 50 countries
with Australia, Canada, China, Peru and the U.S.A. being the
leading producers. Most zinc mines are underground but some new
mines are of the open pit type. 80% of the mines are underground,
8% open pit and the rest a combination of both.
Centuries prior before zinc was discovered in metallic form,
its ores were used for making brass. Brass was produced by the
Romans in the time of Augustas (20 B.C. - 14 A.D.) In 1374, zinc
was recognized in India as a new metal and at Zawar, India, both
zinc metal and zinc oxide were made from the 12th to the 16th
century. Zinc then moved and was manufactured in China in the
17th century and then in 1743 the first European zinc production
was established at Bristol in the United Kingdom. Zinc is the
fourth most widely used metal. After world War II, 46 percent
of zinc are contained in or were produced from deposits discovered
after the war was over, then later on 75% of zinc was produced
in the United States. In 1993 50% of the zinc mined came from
Alaska and Tennessee, New York and Missouri are also top producers
of zinc metal. The US is the worlds largest consumer of zinc.
80% is consumed in slab format while 20% is consumed in compounds.
With a overall rise in metals and minerals prices have rose about
6.3% each year and the nickel and zinc turned in at the top.
Zinc prices continued their climb with result to strong demand
particularly in Asia, we should see prices advance even higher
in the future. |
Back to
top
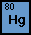 |
Mercury -
Group 15 |
|
|
Element Mercury is also known as the quicksilver. Its
atomic number is 80, and the atomic mass is 200.59 g. It is a
heavy transition metal with a density of 13.534 g/cm³, and
it is liquid at room temperature. The most abundant isotope of
mercury is Hg 202 (29.86%). The most long-lived radioisotope
is Hg 194 with a half life of 444 years. Its electron configuration
is 4f14 5d10 6s2 , and it has 2, 8, 18, 32, 18, 2 in each of
the six energy levels.
Mercury is a naturally occurring element in earth's crust
with an average abundance of 0.08 mg/km. There is a number of
way that the mercury is been produced, such as extracting from
cinnabar ores within the earth's crust, refining natural gases,
government reserves The main producers of mercury are Spain,
China, Kyrgyzstan and Algeria. There are mines in U.S as well,
and they are located in CA, AR, and TX. In 2000, there was 1800
metric tons of mercury produced. The excessive supply of mercury
decreases the price of mercury to $186.04 in 1997. Mercury has
negative effect on the environment and on the human health, therefore
since the mid 1990, 700 to 900 metric ton of recycled mercury
have been produced. The usage of recycled mercury limits mining
which is beneficial to the environment. Not only mercury has
negative effect on environment, its effect on human health also
caused the demand for mercury to decrease. Mercury can cause
damage to the human health because it is toxic to the nervous
system which includes the brain and the spinal cords. The main
cause of toxicity is inhalation of mercury vapors. Mercury exposure
can also harm the heart, kidneys, and the immune system of people
of all ages. If young children exposed to mercury toxicity, it
can decrease their capacity to think and learn.
Despite the negative effect of mercury, it has many good properties
and is used for a variety of purposes. It is used for extraction
of gold and silver, in thermometer, in electronic switches, in
laboratory analyses reactants, etc.
Works Cited
"Basic Information." U.S Environmental Protection
Agency. 29 January 2006. <http://www.epa.gov/mercury/about.htm>.
"Mercury." Wikipedia, The Free Encyclopedia. Wikimedia
Foundation. 29 January 2006. <http://en.wikipedia.org/wiki/Mercury_(element)#Occurrence_in_the_environment>.
"Scientific Facts on Mercury." GreenFacts.org. GreenFacts
Organization. 29 January 2006. http://www.greenfacts.org/mercury/l-3/mercury-5.htm#2. |
Back to
top
 |
Titanium - Wednesday make-up group |
|
|
Worldwide each year, 4221 tons of Titanium is produced.
The primary producers of this element are Australia, South Africa,
Canada, Norway, and Ukraine. Australia produces 30.6%, South
Africa produces 20.1%, Canada produces 18.2%, Norway produces
9.1%, and Ukraine produces 8.5%. Geology is linked to these countries
because of their high concentration of Titanium and their ability
to mine this element.
Titanium is produced by the Kroll process, which is a complex,
expensive batch process developed in 1946 by William Kroll. In
this process, the oxide is converted to chloride through carbochlorination,
where chlorine gas is passed over ilmenite in the presence of
carbon to produce TiCl4. This is condensed and purified then
reduced with 800 degrees C molten magnesium in an argon atmosphere.
I do not have any information about mines in the U.S., but if
there are any I do not think they are a major producer based
on the information in question number 5.
Titanium is widely produced using the Kroll method, however
there have been newer processes being brought into production.
The FFC Cambridge Process may displace the Kroll process. This
new process uses feedstock titanium dioxide powder to make the
end product into powder or a sponge. If mixed oxide powders are
used, the end product is an alloy which costs much less to make.
The van Aikel de Boer process purifies Titanium to ultra high
purity in small amounts. Titanium oxide is also produced by taking
titanium mineral ores and grinding it with potassium carbonate.
With these examples I think it is safe to say that Titanium production
is on an upward trend because of the technology advances in recent
years. |
Back to
top
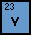 |
Vanadium - Thursday make-up group |
|
|
Vanadium has the symbol V on the periodic table and
has the atomic number 23. It also has an atomic weight of 50.9415,
which means it has 28 neutrons. Vanadium can form metallic bonds.
In fact, it has a minimum oxidation number of -1 and a maximum
oxidation number of 5. This means Vanadium can form oxides such
as VO, V2O3, VO2, V2O5.
Vanadium was discovered in 1801, and the use of it has increased.
This is because it is used often to build objects that are currently
used. Vanadium, which is a silvery grey metallic, can be a solid,
liquid, or gas. It is mostly used to make tools, military vehicles,
and engine parts. It is also used in other forms, such as foil,
powder, and rods. It can also be used to make glass. Vanadium
is useful because it can support massive amounts of weight. Vanadium
can be found in different rocks, such as sandstones, and 65 different
minerals. This means it is an easy element to find while mining.
In addition, Vanadium is found in the Earth s crust, since it
is found in rocks. There is also high amounts of Vanadium in
coal, crude oil, tar sands, and oil shale.
Vanadium is found in the United States, and there is enough
to provide for the US needs. It is sometimes cheaper for the
US to import Vanadium. It can be imported from nations such as
Canada, South Africa, China, and the Czech Republic. Also, Vanadium
is toxic, so it always needs to be used safely. Commercial vanadium,
costs about $20 a pound, and has about 95% purity. It is $100
an oz for 99.9% purity of Vanadium. Vanadium is an important
element to the United States because it is used in everyday life. |
Back to top
Course home page |

